FISH Quality Control Services
As Fluorescent in situ hybridization (FISH) is simple, reliable and cost-effective, FISH is a major technology that is applied in diagnosis, especially for hematologic malignancies, even in the area of next-generation sequencing (NGS). The implementation of FISH in the laboratory requires control with attention to when it is appropriate to apply the technology, a systematic approach to the validation of probes, policies and procedures involved. Knowledge of the limitations of any FISH test is required in relation to the probe and tissue being examined, since errors of analysis and interpretation can result in incorrect management. If you want to know your options for FISH quality control in your lab, Creative Bioarray can help you with this! Creative Bioarray has years of experience in performing FISH, cytogenetic services, we can also provide custom FISH quality control service for your lab’s needs!
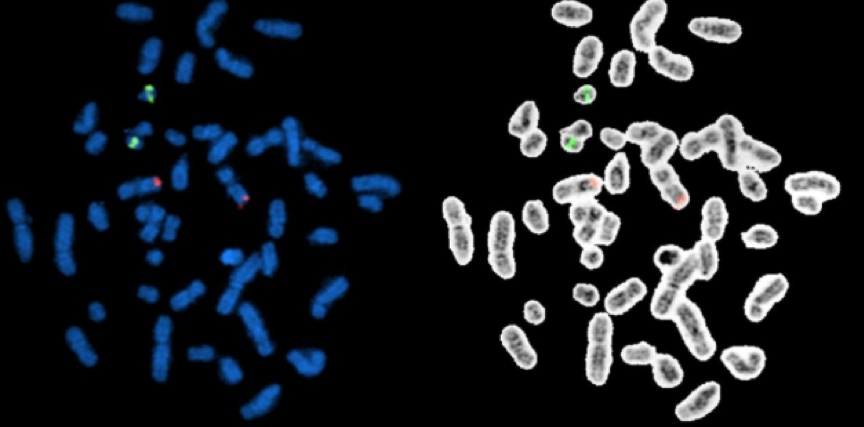 Figure 1. BCR-ABL1 ES FISH probe localization. The ABL1 probe hybridize to the band level 9q34 (red signals) and the BCR probe to the band level 22q11.2 (green signals) on the DAPI banding (metaphases on the left side), which have been sequentially confirmed by inverted G-Banding (metaphases on the right side)
Figure 1. BCR-ABL1 ES FISH probe localization. The ABL1 probe hybridize to the band level 9q34 (red signals) and the BCR probe to the band level 22q11.2 (green signals) on the DAPI banding (metaphases on the left side), which have been sequentially confirmed by inverted G-Banding (metaphases on the right side)
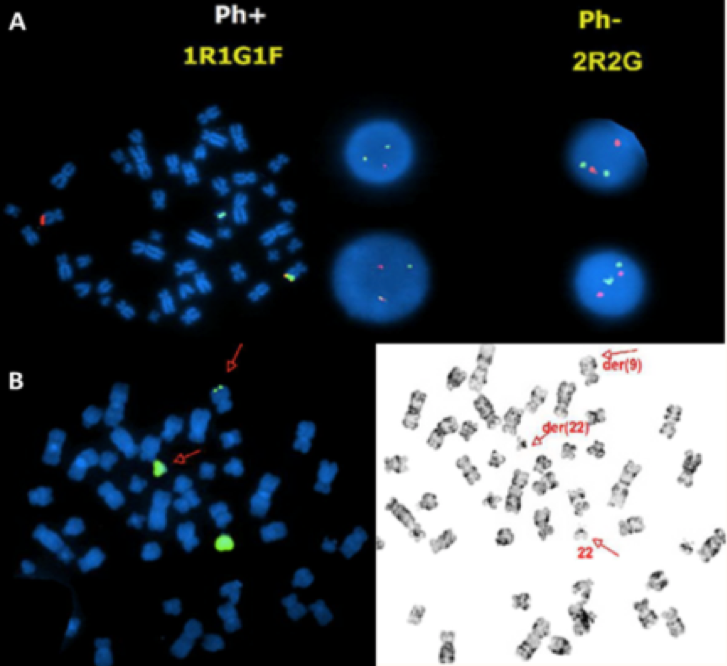 Figure 2. A BCR-ABL1 fusion positive B-ALL case with an unusual signal pattern of 1R1G1F on the interphase FISH images (middle in the top row) and but the fusion signal on the abnormal chromosome 9 on the metaphase FISH image (left in the top row) indicating that the BCR-ABL1 fusion is caused by insertion of part of the BCR gene into the ABL1 gene in this case.
Figure 2. A BCR-ABL1 fusion positive B-ALL case with an unusual signal pattern of 1R1G1F on the interphase FISH images (middle in the top row) and but the fusion signal on the abnormal chromosome 9 on the metaphase FISH image (left in the top row) indicating that the BCR-ABL1 fusion is caused by insertion of part of the BCR gene into the ABL1 gene in this case.
Applications:
- FISH Program Validation
- Probe Localization
- Next Generation Sequencing (NGS) Quality Control
Features:
- High accuracy and sensitivity
- Fast turnaround time
- Competitive pricing
Creative Bioarray offers FISH Quality Control Services for your scientific research as follows:
- We will help you build your own FISH program which will meet your requirements.
- We can offer slides from slide samples from individuals that will meet your different quality control requirements.
- We can offer FISH probe validation, which ensures that the probe targets its intended chromosome regions.
Quotation and ordering
Our customer service representatives are available 24hr a day! We thank you for choosing Creative Bioarray at your preferred FISH Quality Control Services.
References
- Sugita S.; et al. Practical use and utility of fluorescence in situ hybridization in the pathological diagnosis of soft tissue and bone tumors[J]. Journal of Orthopaedic Science, 2017, 22(4): 601-612.
- Niu X.; et al. Anaplastic Lymphoma Kinase Testing: IHC vs. FISH vs. NGS[J]. Current treatment options in oncology, 2017, 18(12): 71.
- Quijada-Álamo M.; et al. Next-generation sequencing and FISH studies reveal the appearance of gene mutations and chromosomal abnormalities in hematopoietic progenitors in chronic lymphocytic leukemia[J]. Journal of hematology & oncology, 2017, 10(1): 83.
- Dubuc A M.; et al. FISHing in the dark: How the combination of FISH and conventional karyotyping improves the diagnostic yield in CpG‐stimulated chronic lymphocytic leukemia[J]. American journal of hematology, 2016, 91(10): 978-983.
- Arber DA.; et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127: 2391-2405.

